MEDICAL
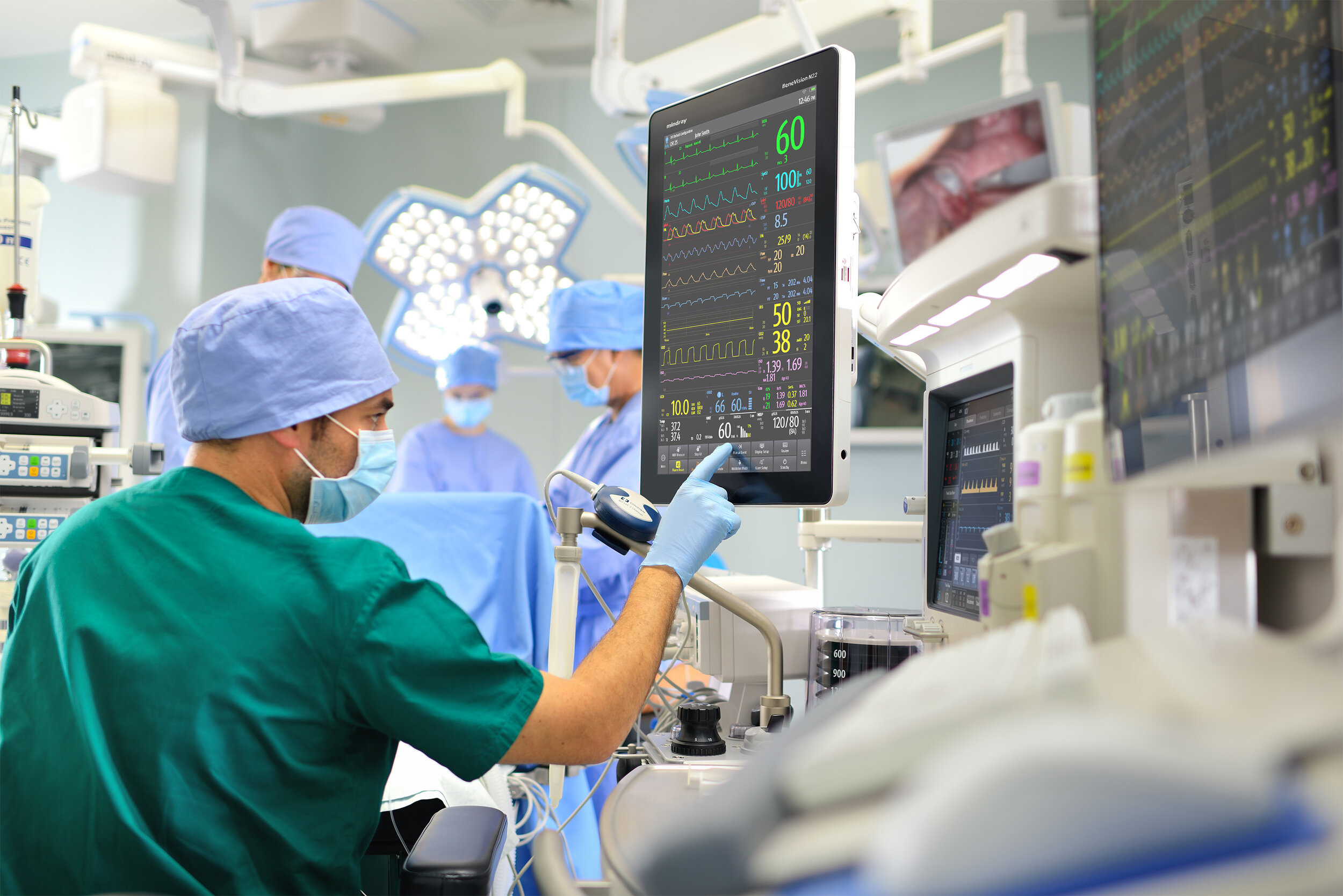
User Centric Design consultants are experienced in the Medical Sector having worked for clients who design and market medical products. Our approach follows best practise in the application of the Usability Engineering Process to ensure products meet international medical devices standards (IEC62366-1) and Food and Drug Administration (FDA) regulatory requirements.
As more of the world aligns itself with these standards we can support regulatory delivery of design evidence in a Usability Engineering File for the UK and other countries.
We apply our Human Factors skill set to:
Deliver your Use Specification
Produce your User Interface Evaluation Plan
Run and write-up your Use Related Risks Analysis and Mitigations
Develop and test Instructions For Use or Surgical Techniques to ensure they are understood and don’t drive use errors into the design
Produce Formative protocols, discussion guides and reports
Run and observe early and late stage Formatives
Produce Summative protocols, discussion guides and reports
Run and observe Summative Studies
Compile the Usability Engineering File
Write the final Usability Engineering Report (FDA)